- Product Details
Keywords
- competitive price Rivaroxaban
- high quality Rivaroxaban
- Rivaroxaban in stock
Quick Details
- ProName: competitive price Rivaroxaban USP
- CasNo: 366789-02-8
- Molecular Formula: C19H18ClN3O5S
- Application: For elective hip or knee replacement o...
- ProductionCapacity: Metric Ton/Day
- Purity: 99%
- LimitNum: 0 Metric Ton
Superiority
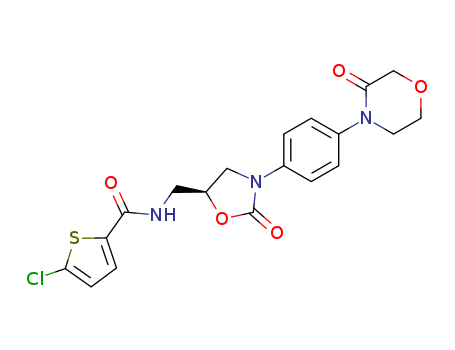
Synonyms:5-Chloro-N-[[(S)-3-(4-(3-oxomorpholin-4-yl)phenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]-thiophene-2-carboxamide;BAY 59-7939;Xarelto;
Li cutting Shaaban and dabigatran is two kinds of antithrombotic drugs is currently the most promising, the essential difference with the traditional anti thrombosis drugheparin is that it does not require the antithrombin III participation, can direct factor Xa antagonist free and combined. There is need for antithrombin and heparin can play a role, and the Xa factor prothrombin complex in the invalid. The two novel oral anticoagulants is the medical profession as a major advance in anticoagulanttherapy and potentially fatal thrombosis prevention field, will become the newmilepost on the history of the development of cardiovascular drugs.
Li cutting Shaaban (Rivaroxaban) by the German Bayer pharmaceuticals and USAJohnson company joint research and development success. In 2008 October, was approved in Canada and the European Union, the trade name Xarelto. LeeShaaban is cutting the first oral direct factor Xa inhibitor can be global, highly selective, competitive inhibition of free and combined factor Xa and prothrombinactivity in a dose dependent manner, prolonged activated partial thromboplastin time plate (PT) and prothrombin time (aPTT), so as to prolong the clotting time,reduce the formation of thrombin. It has high bioavailability, treatment of diseasespectrum is wide, the dose effect relationship stability, convenient oral administration, the characteristics of low bleeding risk.
Drug benefit cutting Shaaban also control the venous thrombosis. Mainly used in the clinical prevention of hip and knee arthroplasty in patients with deep venous thrombosis (DVT) and pulmonary embolism (PE) formation. Can also be used for the prevention of non valvular atrial fibrillation in patients with cerebral stroke andnon central nervous systemic embolism, reduce the risk of recurrent coronary artery comprehensive syndrome etc..
Dabigatran etexilate (Dabigatran) is a new anti clotting drug has manycharacteristics of the German development of Boehringer ingelheim. In 2008 April,the first listed in Germany and the UK, the trade name Pradaxa. This is the secondwarfarin after 50 years to the first listing of the anticoagulant oral drug, is theanticoagulant treatment field and potentially fatal thrombosis prevention fieldanother milepost.
Dabigatran is a new type of synthetic direct thrombin inhibitor, is a pro drug for oralthrombin inhibitor, belongs to non peptide. Oral absorbed by the gastrointestinal,converted in the body to have direct anticoagulant activity of dabigatran. Specificfiber protein drug binding to thrombin binding site, preventing the fibrinogenolyticinto fibrin, which block the final step and thrombus formation of coagulation cascade network






![2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid](http://file1.lookchem.com/cas/reactions/2021/07/21/27036082.png)
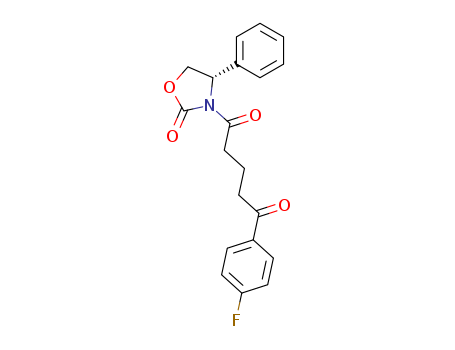
![4S)-3-[(5S)-5-(4-Fluorophenyl)-5-hydroxypentanoyl]-4-phenyl-1,3-oxazolidin-2-one](http://file1.lookchem.com/cas/reactions/2021/06/30/9079993.png)